Helping Patients Transition After the FDA Ban on Compounded GLP-1s
Helping Patients Transition After the FDA Ban on Compounded GLP-1s
- Jason K
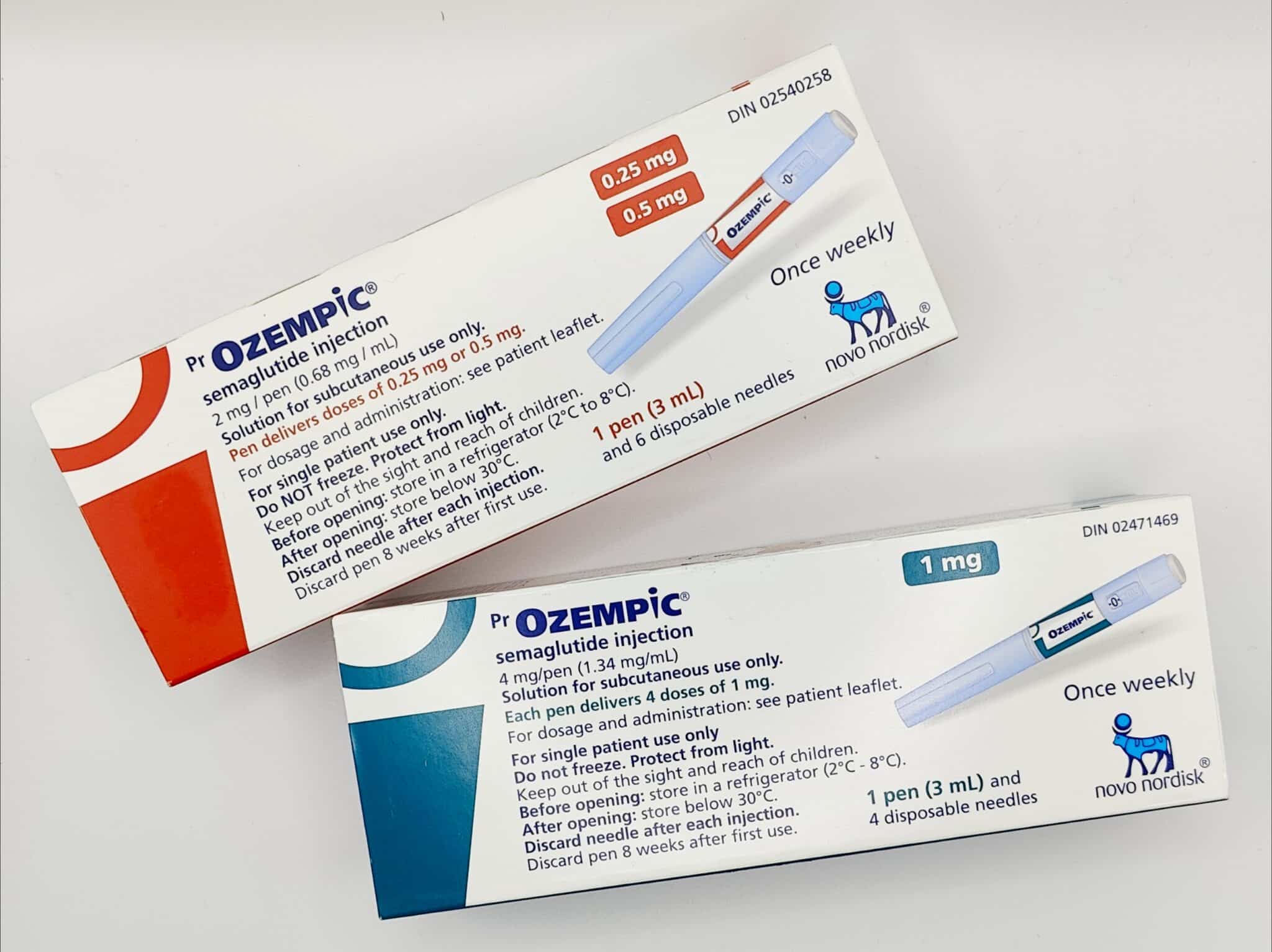
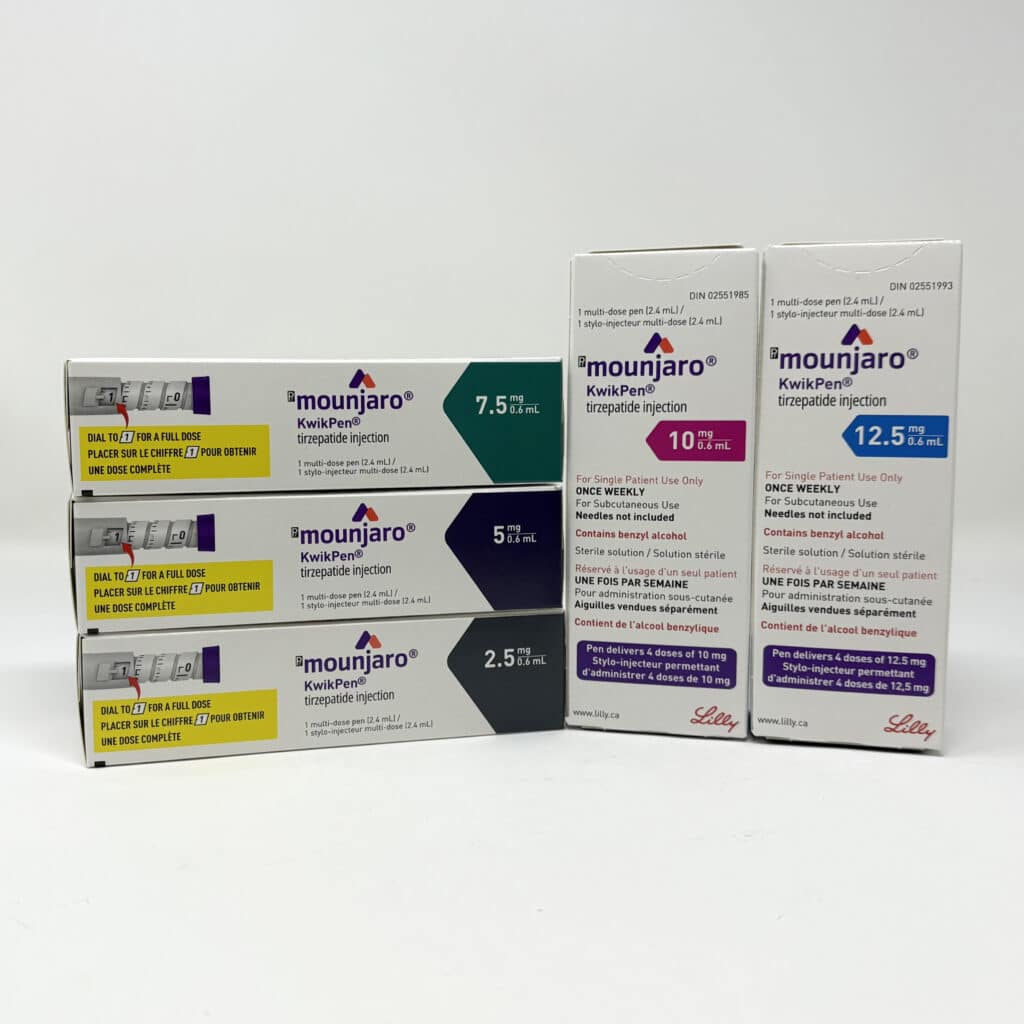
The FDA’s upcoming ban on compounded GLP-1 medications, set to take effect in May 2025, will have a significant impact on both medical clinics and their patients. For many individuals who have been relying on compounded versions of Ozempic, Wegovy, and Mounjaro, this change means they will need to switch to FDA-approved medications.
Medical clinics that previously prescribed compounded semaglutide or tirzepatide must now prepare to help their patients transition to brand-name alternatives. This article will discuss why the FDA is banning compounded GLP-1s, how clinics can support their patients, and what options remain available for those concerned about costs.
Why Is the FDA Banning Compounded GLP-1s?
Compounded versions of semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro) were allowed temporarily due to shortages of the brand-name drugs. Pharmacies were permitted to mix and sell compounded versions to meet the overwhelming demand for GLP-1 medications used for diabetes and weight loss.
However, as the supply of brand-name GLP-1 medications stabilizes, the FDA has determined that compounded versions are no longer necessary and will ban their sale starting May 22, 2025.
How Medical Clinics Can Support Patients
Clinics that have been prescribing compounded GLP-1s will need to help patients navigate this transition to avoid disruptions in their treatment plans. Here are several ways clinics can provide guidance:
1. Educate Patients on the Ban and Their Options
Many patients may not yet be aware that compounded versions of Ozempic, Wegovy, and Mounjaro will no longer be available after May 2025. Clinics should proactively inform patients about the upcoming changes, explain why the ban is happening, and discuss what it means for their treatment.
Patients should understand that they will need to switch to an alternative GLP-1 medication to continue receiving the same level of treatment for diabetes or weight loss.
2. Guide Patients Through the Transition to Brand-Name Medications
For those currently on compounded GLP-1s, transitioning to a brand-name alternative will be necessary. Clinics can help by:
- Assessing patient needs: Some patients may need a dose adjustment when switching from compounded medications to Ozempic, Wegovy, or Mounjaro.
- Prescribing brand-name medications: Ensure patients receive a prescription for the appropriate approved alternative.
- Monitoring for side effects: Brand-name medications may have different formulations than their compounded counterparts, so patients should be closely monitored during the switch.
3. Address Cost Concerns and Offer Alternative Solutions
One of the main reasons patients opted for compounded GLP-1 medications was cost savings. Many clinics will need to help patients find affordable options for transitioning to brand-name medications without dramatically increasing their expenses.
Options include:
- Exploring lower-cost options: For those paying out-of-pocket, an alternative option would be using an online Canadian prescription referral service like Over the Border Meds, which may provide significant savings on brand-name GLP-1s.
Why Brand-Name GLP-1s Are More Reliable Than Compounded Versions
While compounded versions of Ozempic, Wegovy, and Mounjaro provided an option during shortages, brand-name medications offer more consistency and safety:
- Strict quality control: Health Canada and FDA-approved medications undergo rigorous testing for purity, potency, and effectiveness, while compounded versions may vary in quality.
- Reliable dosing: Brand-name GLP-1s come in standardized dosages, reducing the risk of variability seen with compounded versions.
- Reduced side effect risk: Some compounded formulations contained salt-based semaglutide, which has not been tested for long-term safety like Health Canada/FDA-approved versions.
For patients who were hesitant to switch to Ozempic, Wegovy, or Mounjaro, clinics can reassure them that brand-name medications offer more predictable results and a safer treatment experience.
How Clinics Can Prepare for the Transition
To avoid disruptions in patient care, clinics should begin preparations now for the upcoming changes. Steps to take include:
- Reviewing patient lists: Identify those currently using compounded GLP-1s and schedule appointments to discuss their transition plan.
- Training staff: Ensure healthcare providers and support staff understand the FDA ban and can effectively communicate alternative options to patients.
- Updating prescription protocols: Adjust internal prescription practices to facilitate switching patients to brand-name alternatives in a timely manner.
- To look for alternative pharmacy options, such as using an online Canadian prescription referral service, which offers the same brand name product at a fraction of the cost.
Final Thoughts
The FDA ban on compounded GLP-1 medications will require clinics to take a proactive role in helping patients transition to Ozempic, Wegovy, or Mounjaro. By educating patients, guiding them through the switch, and addressing cost concerns, clinics can ensure that their patients continue receiving the care they need without unnecessary disruptions.
For clinics seeking affordable alternatives for their patients, prescription referral services like Over the Border Meds offer an opportunity to help patients access brand-name medications at a lower cost. Planning ahead will be essential in helping patients successfully move from compounded GLP-1s to safe, approved alternatives.