Tresiba FlexTouch Insulin
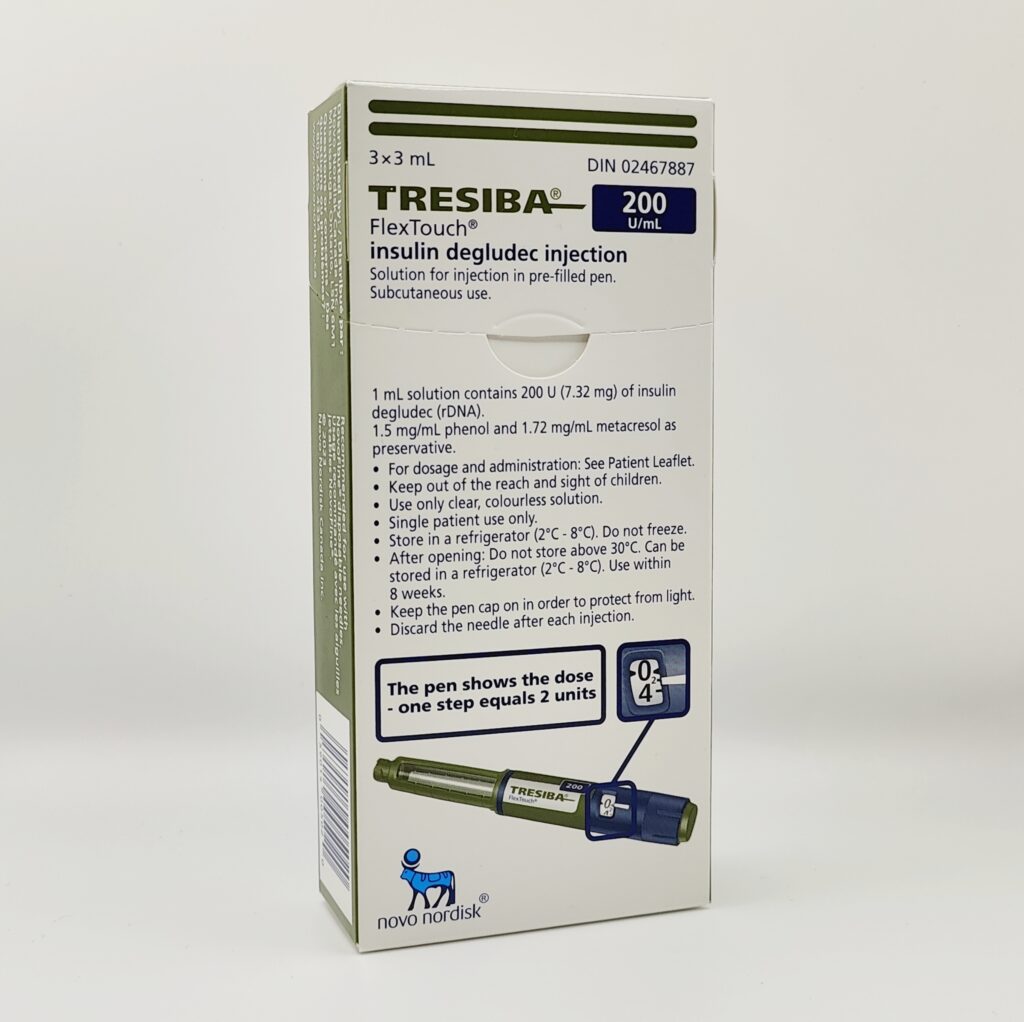
Tresiba FlexTouch Insulin
Insulin degludec
Product information
Buying Tresiba insulin from Canada offers a more affordable option for managing diabetes, with the same high-quality standards as U.S. medications. Use coupon code HELLO10 to save 10% on your first order of Tresiba insulin and take advantage of added savings.
RX Required
Product options
Tresiba (Insulin Degludec) FlexTouch Insulin Pens
What are Tresiba FlexTouch Insulin Pens?
Tresiba FlexTouch insulin pens contain insulin degludec, a long-acting basal insulin designed to manage blood sugar levels in adults and children with type 1 and type 2 diabetes. With its ultra-long duration of action—lasting over 42 hours—Tresiba helps maintain steady blood sugar levels with just one injection per day. The FlexTouch design simplifies administration, allowing for precise dosing without manual vial preparation, making it an ideal choice for patients seeking convenience and efficacy.
Tresiba’s extended action reduces the risk of blood sugar fluctuations and minimizes the chances of hypoglycemia, particularly overnight, providing peace of mind for diabetes patients. This innovative formulation also allows for flexible dosing times, offering added convenience for busy lifestyles.
How Tresiba Works
Tresiba mimics the body’s natural insulin response by enabling glucose absorption into cells and reducing excessive blood sugar levels. Its unique molecular structure forms a depot under the skin after injection, providing a consistent and gradual release of insulin. This steady action supports optimal glucose control throughout the day and night. Tresiba is particularly effective for patients who need stable, predictable insulin coverage with fewer peaks and troughs.
Unlike some other basal insulins, Tresiba’s flexibility allows for dosing adjustments, ensuring patients can administer their insulin at different times of the day, provided there’s at least an 8-hour gap between doses.
Tresiba FlexTouch Dosage and Administration
The Tresiba FlexTouch pen is available in two concentrations: 100 U/mL and 200 U/mL. The 200 U/mL option is particularly beneficial for patients requiring higher daily doses, as it reduces the number of injections needed.
The typical starting dose for insulin-naive adults with type 2 diabetes is 10 units once daily. For type 1 diabetes, Tresiba is used alongside mealtime (bolus) insulin to achieve comprehensive blood sugar control. Dosages are individualized based on factors such as blood sugar monitoring results, dietary habits, and your healthcare provider’s recommendations.
To administer Tresiba, inject it subcutaneously into the abdomen, thigh, or upper arm, rotating injection sites to prevent complications such as lipodystrophy. Always follow your healthcare provider’s instructions regarding timing and dosing.
Possible Side Effects of Tresiba
Like all medications, Tresiba may cause side effects. Common side effects include:
- Hypoglycemia (low blood sugar), often marked by symptoms such as dizziness, sweating, and confusion
- Injection site reactions, such as pain, redness, or swelling
- Weight gain
Less common but more serious side effects include:
- Severe hypoglycemia, which may require immediate medical attention
- Allergic reactions, including rash, itching, or anaphylaxis
- Lipodystrophy (changes in fat tissue at injection sites)
If you experience severe symptoms, consult your healthcare provider immediately.
Drug Interactions
Tresiba may interact with other medications, potentially affecting its efficacy or increasing the risk of side effects. Medications to be cautious of include:
- Oral antidiabetic drugs, such as metformin or sulfonylureas
- SGLT-2 inhibitors, which also lower blood sugar levels
- ACE inhibitors and angiotensin II receptor blockers (ARBs), which may increase insulin sensitivity
- Beta-blockers, which can mask symptoms of hypoglycemia
To ensure safety, always inform your healthcare provider about all medications, supplements, and herbal products you are taking.
Precautions When Using Tresiba FlexTouch Insulin Pens
Before starting Tresiba, inform your healthcare provider if you:
- Have a history of kidney or liver problems
- Frequently experience episodes of low blood sugar
- Are pregnant, planning to become pregnant, or breastfeeding
Patients with these conditions may require dosage adjustments or additional monitoring while using Tresiba.
Buying Tresiba FlexTouch Insulin Pens from Canada
For U.S. patients, purchasing Tresiba FlexTouch pens from Canada offers significant cost savings compared to U.S. prices. Here’s how to ensure a safe and affordable purchase:
- Check Pharmacy Accreditation: Purchase from licensed Canadian pharmacies certified by organizations like the Canadian International Pharmacy Association (CIPA).
- Consult Your Doctor: Discuss your plan with your healthcare provider to ensure it aligns with your treatment goals.
- Provide a Valid Prescription: A U.S. prescription is required to buy Tresiba from a Canadian pharmacy.
- Understand Import Rules: The FDA allows a 90-day supply of medication to be imported for personal use.
Buying from Canada provides access to the same high-quality medication at a fraction of the cost.
Storage Instructions for Tresiba FlexTouch Insulin Pens
Unopened Tresiba pens should be stored in the refrigerator at 2°C to 8°C (36°F to 46°F). Once opened, they can be kept at room temperature (below 30°C/86°F) for up to 8 weeks. Avoid exposing the pens to heat, freezing temperatures, or direct sunlight. Always keep Tresiba out of reach of children and pets.
Frequently Asked Questions (FAQs)
How long does Tresiba last after opening?
Once opened, Tresiba FlexTouch pens can be used for up to 8 weeks when stored at room temperature (below 30°C/86°F).
Can I buy Tresiba FlexTouch pens from Canada without a prescription?
No, a valid prescription is required to purchase Tresiba from a Canadian pharmacy.
Is Tresiba suitable for type 1 diabetes?
Yes, Tresiba is often prescribed for type 1 diabetes in combination with a rapid-acting insulin to manage mealtime blood sugar levels.
For more information, consult your healthcare provider or refer to the official product monograph here.