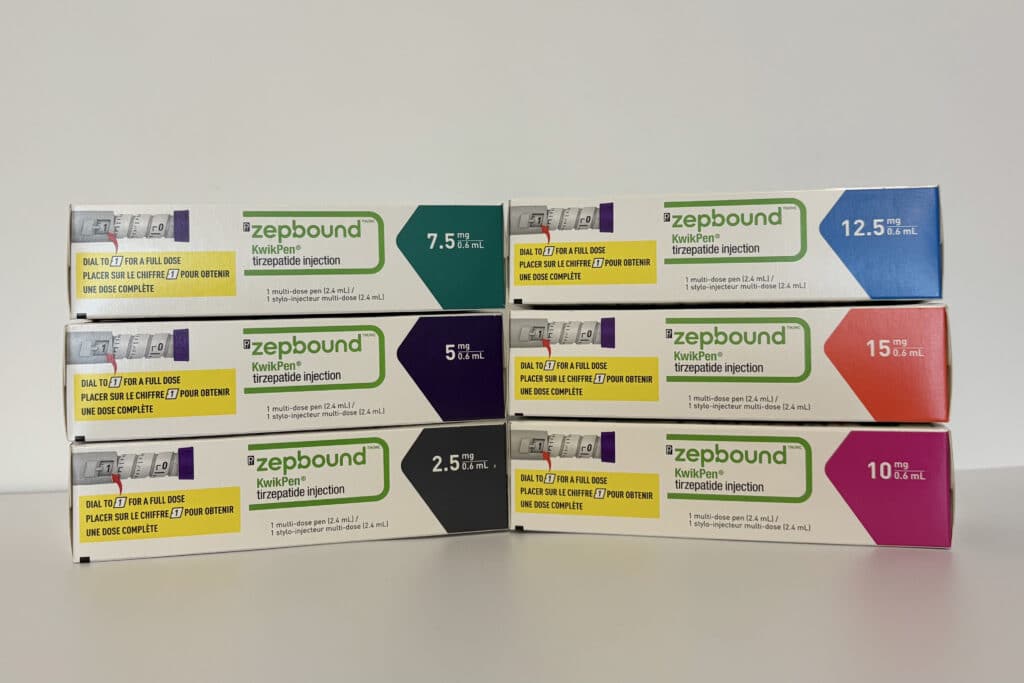
Health Canada approved Zepbound in early 2025, marking a major step forward for access to tirzepatide specifically for weight management. While approval is a key milestone, it doesn’t automatically mean the medication will be on pharmacy shelves right away. Distribution, pricing negotiations, and formulary listings can take time. That said, many expect availability to follow relatively quickly, with Canadian pharmacies likely offering Zepbound as early July 15, 2025. When it does launch, it will likely be available in the familiar KwikPen format, multi-dose, prefilled injection pens similar to those already used for Mounjaro.
What Zepbound in Canada Means for U.S. Patients: Availability, Cost, and How to Access It
What Zepbound in Canada Means for U.S. Patients: Availability, Cost, and How to Access It
Zepbound (tirzepatide), the brand-name GLP‑1 medication for weight loss, has recently been approved by Health Canada, expected to be available in pharmacies any day. For American patients paying cash for GLP‑1s, this marks an exciting opportunity to access the treatment and potentially more affordably.
This guide explains what the Canadian launch means, expected pricing, import rules, and how U.S. patients can prepare now to access Zepbound once it’s available north of the border.
What Is Zepbound and How Does It Differ from Mounjaro?
Zepbound and Mounjaro both contain tirzepatide, a dual GIP/GLP‑1 receptor agonist. The key differences are:
- Zepbound is marketed specifically for weight management (15 mg dose), while Mounjaro is primarily for diabetes
- Both use the same active ingredient, but can differ in packaging and dosing pens.
- In Canada, Mounjaro has been available for diabetes since 2022; Zepbound’s approval now expands options for patients seeking higher-dose weight loss treatment.
When Will Zepbound Be Available in Canada?
Expected Pricing in Canada vs. the U.S.
In the U.S., Zepbound pens retail pricing starts around $1,000–$1,300 per month, depending on dosage.
Canadian pricing isn’t final yet, but based on existing trends, like Wegovy and Mounjaro, prices are expected to be 30–50% lower than U.S. rates.
If Canadian pricing follows Wegovy/mounjaro patterns, Americans could see significant savings once Zepbound is available north of the border.
Is It Legal to Import Zepbound from Canada?
Yes. U.S. law allows individuals to import up to a 90-day supply of prescription medication for personal use, provided:
- You have a valid U.S. prescription
- The medication is shipped directly to your home (not for resale)
Once Zepbound is available in Canada, you’ll be able to order it legally through licensed Canadian pharmacies using prescription referral services.
Who Stands to Benefit Most?
- U.S. patients paying out-of-pocket for GLP‑1 weight-loss medications
- Individuals not covered for weight-loss drugs by insurance
- Those who want the higher 15 mg dose available with Zepbound, versus lower-dose options
- Patients already familiar with tirzepatide who would prefer continuity in medication
How to Prepare Now
If you’re considering ordering Zepbound from Canada, here are some steps to take:
1. Talk to your provider about writing a prescription for Zepbound (15 mg) in advance
2. Monitor Canadian pharmacy programs and pricing updates for official launch
3. Use prescription referral services to prepare for smooth ordering once available
4. Gather any necessary documentation: U.S. prescription, import forms, etc.
5. Understand storage and shipping requirements (e.g., refrigeration)
Things to Know Before Switching
- Canadian pens may look different than U.S. versions, it’s still the same medication
- Availability may be limited initially; orders may take longer than usual
- The regulatory pathway in Canada ensures Health Canada-approved quality standards
- Once available, expect a choice between single-use KwikPens and multi-use formats, similar to existing products.
Pharmacy Wholesalers Are Already Preparing for Zepbound Launch
While Zepbound has only recently received Health Canada approval, pharmacy suppliers and wholesalers in Canada have already begun listing Zepbound KwikPens as “coming soon” in their ordering systems. This is often one of the earliest signals that commercial availability is just around the corner.
When suppliers begin assigning product codes and preparing for distribution, it typically means that final logistics, such as packaging, pricing agreements, and shipment scheduling, are underway. Pharmacies often rely on these listings to plan their inventory and ensure patients can access new medications as soon as they are officially released.
The early appearance of Zepbound KwikPens in supplier databases suggests that a late summer 2025 release timeline is realistic. Patients and prescribers looking to access the medication soon after launch should monitor updates closely and prepare prescriptions in advance, especially if they are planning to order from Canada once the product becomes available.
Final Thoughts
The approval of Zepbound in Canada marks a promising development for U.S. patients paying out of pocket for GLP‑1 weight-loss therapy. With likely cost savings, high-dose availability, and import pathways, there’s a real opportunity ahead.
To stay ahead, speak with your provider, watch for official Canadian launch announcements, and get your prescription ready. When Zepbound officially hits Canadian pharmacies, likely in late summer 2025, you’ll be ready to explore a safer, more affordable path to treatment.
Disclaimer: This article is for informational purposes only and not medical advice. Consult a licensed healthcare provider before starting or changing medications. Use reputable, licensed pharmacies to ensure safety.